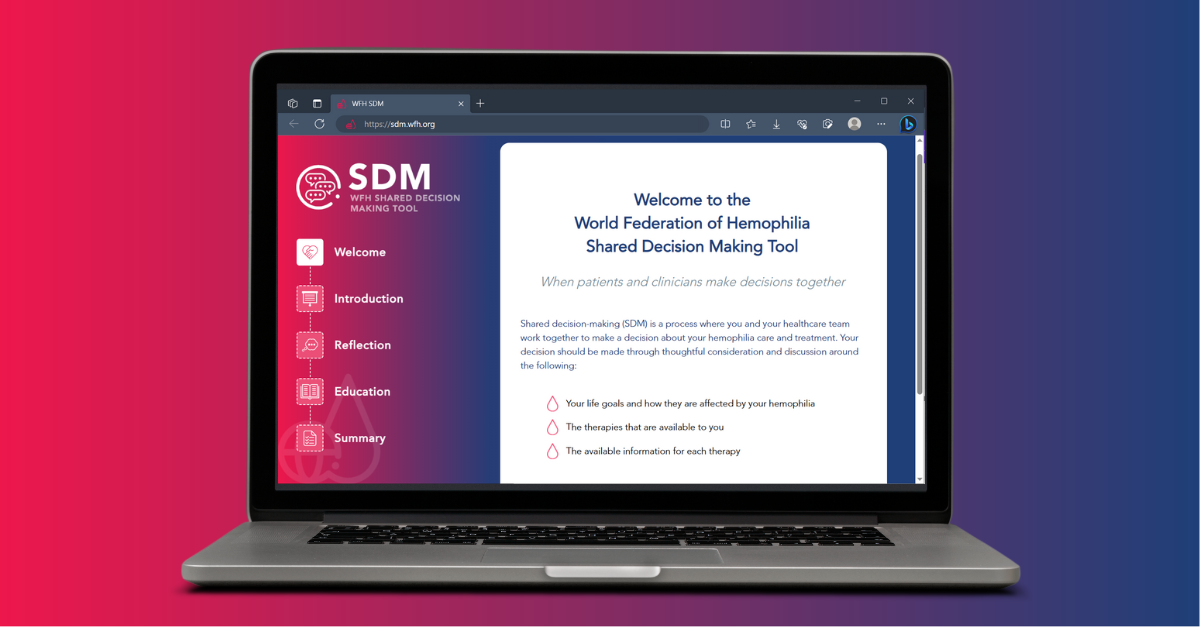
The WFH Shared Decision Making (SDM) Tool is a web-based interactive decision-support system designed to assist PWH and their caregivers who are considering changes in their current treatment regimen. Users are guided through a series of value-based questions they can consider. They are also provided with evidence-based educational content on five prophylactic treatment classes for hemophilia. This living tool is updated twice per calendar year, ensuring that users always have access to the most current information.
In early 2024, the WFH conducted a three-part webinar series focused on shared decision-making for hemophilia. These sessions covered the assessment of risks and benefits for different treatment classes, and highlighted the advantages of using the WFH SDM Tool for both PWH and healthcare providers. Additional training sessions were also held regionally, including an in-person training held in Bogota, Colombia, and sessions at the WFH 2024 World Congress—further emphasizing the tool’s value and application.
Looking ahead, a pilot study is in development to assess the effectiveness of the WFH SDM Tool in real-world settings. This study aims to provide insights into how the WFH SDM Tool supports collaborative decision-making among key stakeholders, and its broader impact on the hemophilia community.
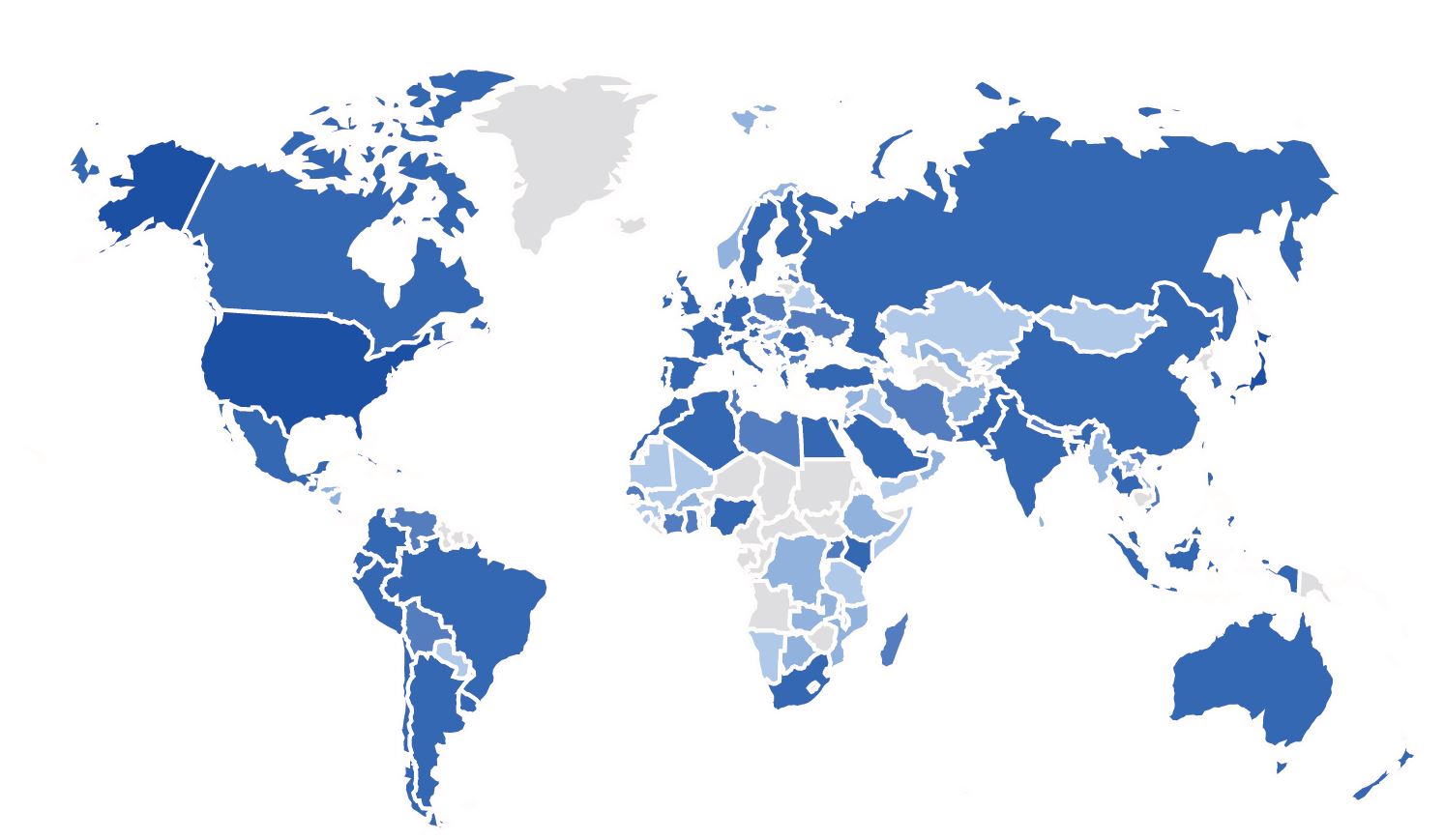
Currently available in English, French, Spanish, Japanese, German and Dutch, with additional translations planned for the future, the WFH SDM Tool is intended to be accessible to a global audience. From August 1, 2023, to August1, 2024, the tool attracted over 8,500 unique visitors from 138 countries, reflecting its growing influence and reach.
As we celebrate this milestone, the WFH invites everyone to explore the potential of shared decision-making in enhancing hemophilia care.
To find out more about shared decision-making for hemophilia treatments, visit the WFH eLearning platform. To access the WFH SDM Tool, click here for a web-based version, or here for a PDF version.
The WFH thanks our Corporate Partners BioMarin Pharmaceutical Inc., CSL Behring, F. Hoffmann-La Roche, Novo Nordisk, Pfizer, Sanofi and Sobi.